Development of nanomaterials that can be concurrently used as MRI contrast agents and brain cancer hyperthermia treatment materials
- Showing improved efficient brain cancer diagnosis and excellent anticancer effects of hyperthermia treatment than existing nanomaterials
- Confirming stability and diagnostic/therapeutic performance, suggesting new cancer treatment directions through hyperthermia treatment
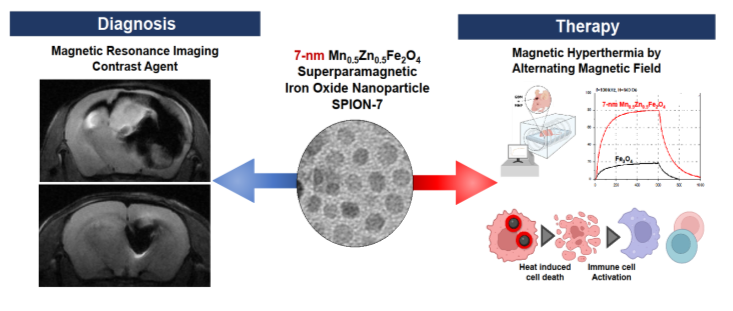
[Figure 1] Brain cancer diagnostic effect (left) and therapeutic effect through high-temperature heating production (right) of 7nm manganese-zinc-iron oxide magnetic nanomaterial (SPION-7) as a contrast agent
A domestic research team has recently developed a highly efficient ‘nanomaterial (MnZn-SPION-7)’ that can be concurrently used as an MRI contrast agent and a brain cancer hyperthermia treatment agent. This material is a 7 nm-sized manganese-zinc-iron oxide (Mn0.5Zn0.5Fe2O4) magnetic nanomaterial that has enhanced MRI contrast ability and hyperthermia treatment effect compared to existing materials. Since it can diagnose and treat cancer simultaneously, this innovative material is expected to play a significant role in developing new cancer treatment methods.
On April 7, a joint research team consisting of Professor Na Yi Rang of the Department of Convergence Medicine & Professor Paek Sun Ha of the Department of Neurosurgery at Seoul National University Hospital, Professor Park Won Cheol of the Graduate School of Convergence Science and Technology at Seoul National University, and Professor Ling Daishun from Shanghai Jiao Tong University announced the results of a study in which they developed a nanomaterial (MnZn-SPION-7) and confirmed its diagnostic and therapeutic effects on glioblastoma through in vivo experiments.
Brain cancer (glioblastoma) is the most common primary malignant brain tumor in adults and is characterized by strong resistance to existing treatments such as chemotherapy and radiotherapy. Although there have been recent advancements in treatment options, such as temozolomide and concurrent chemoradiation, the median survival period for patients with glioblastoma remains less than 15 months.
Since the emergence of magnetic hyperthermia utilizing nanomaterials, iron oxide nanomaterials (SPIONs) have been developed. However, challenges such as the requirement for a high alternating magnetic field (AMF), dosage limitations, and suboptimal imaging contrast have hindered their practical applications. In response, methods of synthesizing other atoms into existing SPIONs have been attempted, but there has been little research that has efficiently optimized both MRI contrast agent effects and anticancer treatment effects through precise atomic doping.
*Alternating magnetic field (AMF): A magnetic field that periodically changes between intensity H and reverse intensity -H over time and interacts with magnetic nanoparticles to generate heat. For example, an alternating magnetic field of 100 kHz alternates between the N and S poles 100,000 times per second.
Accordingly, the research team developed a 7-nm-sized Mn0.5Zn0.5Fe2O4 iron oxide nanomaterial ‘MnZn-SPION-7’ by doping manganese (Mn) and zinc (Zn) into existing iron oxide through a high-temperature pyrolysis manufacturing method, and manufactured PEG-MnZn-SPION-7 by modifying the surface with a methoxy-PEG-silane solvent to improve safety. Afterwards, in vivo and in vitro experiments were conducted to evaluate stability, biosafety, biocompatibility, and diagnostic and therapeutic performance of the nanomaterial, and compared it with existing Fe3O4 iron oxide nanomaterials (SPIONs).
As a result of the study, PEG-MnZn-SPION-7 was uniform in size and shape, and maintained magnetic properties in aqueous solution, proving the stability of the nanomaterial. In addition, it showed a significantly higher MRI T2 enhancement effect than the existing SPION, confirming its excellent performance as a contrast agent.
In terms of therapeutic effect, MnZn-SPION-7 showed more than 5 times higher heat production than the existing SPION in an alternating magnetic field of 140 Oe and 100 kHz (78.4 °C vs. 15.4 °C). In addition, when PEG-MnZn-SPION-7 was injected into glioblastoma cells, the temperature increased by 26.6 °C, causing the glioblastoma cells to disappear, and when the same alternating magnetic field was applied 6 times over 2 weeks, immune cell activation was induced.
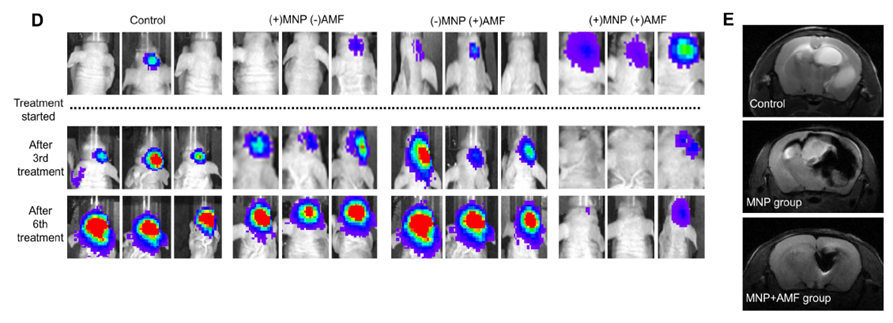
[Figure 2] Elucidation of imaging contrast ability and therapeutic effect in animal experiments: (D) (+) MNP (nanomaterial), (+) AMF experimental group showed greater imaging contrast ability and therapeutic effect than the control group (E) MRI can accurately identify the location and movement path of brain cancer
Professor Na Yi Rang (Department of Convergence Medicine) of Seoul National University Hospital said, “MnZn-SPION-7 nanomaterials enable not only very high-temperature hyperthermia applications but also tumor tracking as an MRI contrast agent at the same time.” She added, “We expect this to be of great help in developing new cancer treatments.”
Professor Park Won Cheol (Department of Applied Bioengineering) of Seoul National University’s Graduate School of Convergence Science and Technology said, “In the future, we plan to develop high-efficiency magnetic nanomaterials and add drug delivery functions through various activations of surface modifications to improve patient-tailored diagnosis and treatment.”
Seoul National University Hospital Professor Paek Sun Ha (Department of Neurosurgery) said, “We expect that cancer treatment using MnZn-SPION-7 nanomaterials could be a very good treatment alternative in patients with not only brain cancer (glioblastoma), but also systemic cancers such as lung cancer, breast cancer, liver cancer, prostate cancer, and skin cancer, who experience relapse despite existing cancer treatments.”
This study was published in the latest issue of the international academic journal ‘Theranostics (IF: 12.4)’.
[Pictures from left] Professor Na Yi Rang from the Department of Convergence Medicine & Professor Paek Sun Ha from the Department of Neurology at Seoul National University Hospital, Professor Park Won Cheol from Advanced Institute of Convergence Technology at Seoul National University, and Professor Ling Daishun from Shanghai Jiao Tong University