Seoul National University Hospital, the world's first to identify the pathological mechanism causing the dilated cardiomyopathy (D-CMP)
- Latrophilin-2, plays a key role in mitochondrial function and cell junction between cardiomyocytes in the myocardium
- Deletion of Latrophilin-2 causes heart failure; an important foundation for developing therapeutic agents
A research team at Seoul National University Hospital recently announced that it has become the first to identify a new pathological mechanism causing 'dilated cardiomyopathy' due to the deletion of latrophilin-2. This has laid an important foundation for developing therapeutic agents for heart failure. According to the study, latrophilin-2 is crucial in regulating mitochondrial function and the cell junction between cardiomyocytes in the myocardium. Therefore, its deficiency leads to decreased cardiac function and heart failure.
Professor Kim Hyo-Soo's research team at the Seoul National University Hospital Biomedical Research Institute (Prof Cho Hyun-Jai, Department of Cardiology, and Dr Kang Minjun, Department of Cardiovascular Research) announced that they discovered a new pathophysiology of dilated cardiomyopathy by creating a 'cardiomyocyte-specific latrophilin-2 deficiency mouse model*' induced by tamoxifen and analyzing its phenotypes.
*It took two years to develop this transgenic mouse model, which was designed to selectively knock out Lphn2 genes in cardiomyocytes using tamoxifen
Cardiovascular disease is a disease that causes the greatest socioeconomic burden worldwide, and it occurs especially in elderly patients over 60 years, leading to heart failure due to decreased contractile function of the heart. Dilated cardiomyopathy is a representative form of heart failure, and currently, only auxiliary drug treatment exists, and there is no fundamental treatment that induces regeneration of cardiomyocytes.
Professor Kim Hyo-Soo's research team (Prof Cho Hyun-Jai and Dr Lee Choon-Soo) discovered five years ago for the first time that Latrophilin-2 is selectively expressed in cardiac progenitors, and that when it is absent, abnormal heart development occurs during embryonic development, resulting in embryonic lethality, demonstrating that this gene is essential for life. This study was carried out to determine the role of Latrophilin-2 in the adult heart. It took two years to create a transgenic mouse model, and the results of two years of characterization were presented.
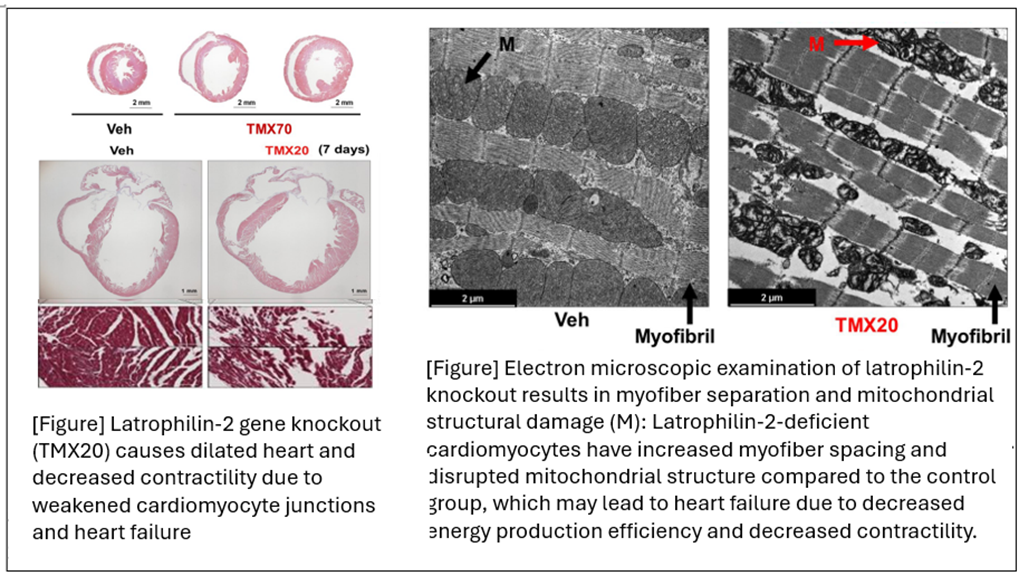
The study results showed that mice that had been administered Tamoxifen to remove Latrophilin-2 from their cardiomyocytes suddenly died within a few days, and the mortality rate was significantly higher than the control group in the myocardial infarction model. Electrocardiogram monitoring indicated that arrhythmia and atrioventricular conduction block were the causes of death. However, surprisingly, a gross autopsy showed that the heart was greatly enlarged, and a tissue examination showed that the myocardial fibers were disjointed and disordered. In addition, an electron microscope examination showed that not only the myocardial fibers were disintegrated, but also the mitochondria within the cells were destroyed. When the heart was thawed, single myocardial cells were isolated, and mitochondrial function was analyzed, it was confirmed that the mitochondrial membrane potential was reduced, which led to the accumulation of reactive oxygen species (ROS).
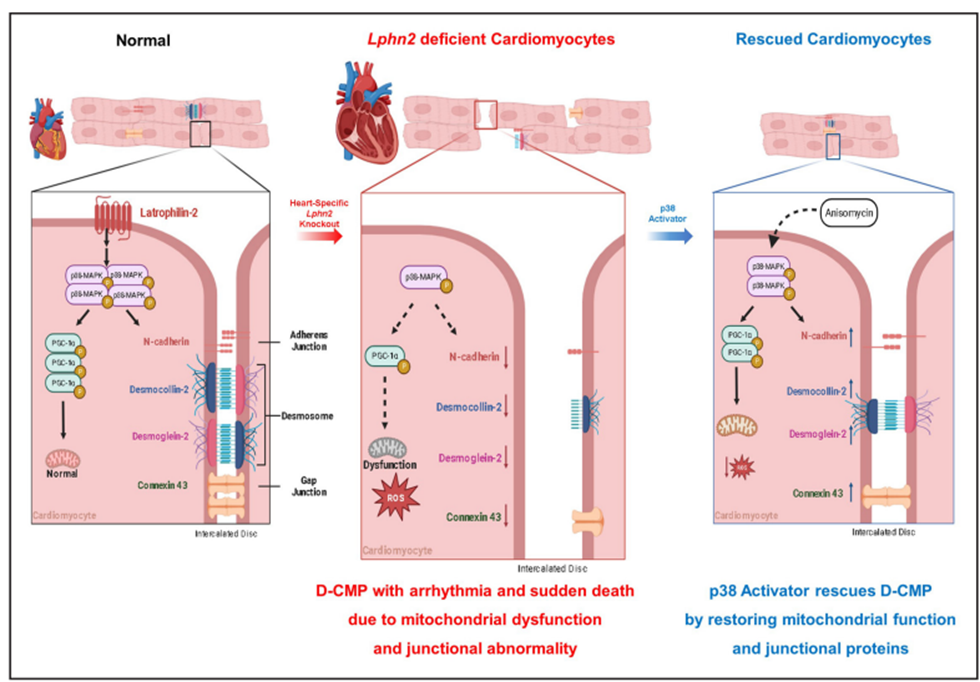
[Figure] Graphical summary for the mechanism of the dilated cardiomyopathy (D-CMP) phenotype in cardiomyocyte-specific, tamoxifen-inducible Lphn2 knockout (Lphn2icko) mice, and rescue of D-CMP by p38 activator
Analysis of the mechanism of this phenomenon showed that when Latrophilin-2 is deficient, the activity of the p38-MAPK pathway decreases, resulting in a decrease in the expression of Adherens, Desmosomes, and Connexins, which are adhesion factors between cardiomyocytes. This leads to disintegration of cardiomyocytes. At the same time, the expression of PGC-1α, a mitochondrial regulatory protein, decreases, which reduces the number of mitochondria and destroys their structure, lowering the efficiency of energy production and worsening cardiac function.
To prove this pathophysiology, the research team confirmed that dilated cardiomyopathy in Latrophilin-2-deficient mice was cured by administering a ‘p38-MAPK pathway activator.’ This proved that heart failure caused by Latrophilin-2 deficiency can be improved, and it is expected to serve as an important foundation for the development of future heart failure treatments.
Prof Kim Hyo-Soo, SNUH Biomedical Research Institute, said, “Since the heart must maintain a continuous beat, the physical coupling between the function of mitochondria and cardiomyocytes is very important,” and “Through this study, we proved that when Latrophilin-2 is deleted in cardiomyocytes, these functions and structures are impaired, which can lead to heart failure and sudden death.”
He continued, “Because the gene sequences of Larophilin-2 are almost identical between mice and humans, the results of this study can also be applied to humans,” adding, “We are currently developing a Latrophilin-2 gene therapy, a ligand that can stimulate the cell surface receptor called Latrophilin-2, as a therapeutic agent.”
This study was conducted with the support of the Ministry of Health and Welfare's Research-oriented Hospital Project and the National Research Foundation of Korea's Biomedical Technology Development Project, and the results were published in the latest issue of 'Circulation Research' (IF: 16.5), the most authoritative journal in the field of basic cardiovascular research.

[Picture from left] Prof Kim Hyo-Soo, SNUH Biomedical Research Institute,
Prof Cho Hyun-Jai, Department of Cardiology, and Dr Kang Minjun, Biomedical Research Institute Cardiovascular Research Group