Seoul National University Hospital develops the World's Fastest Antimicrobial Susceptibility Testing Technology
- Complete antimicrobial susceptibility testing within 13 hours by skipping the blood culture stage—48 hours faster than current methods.
- Expected to improve the prognosis of Sepsis patients and establish a new standard for Sepsis treatment
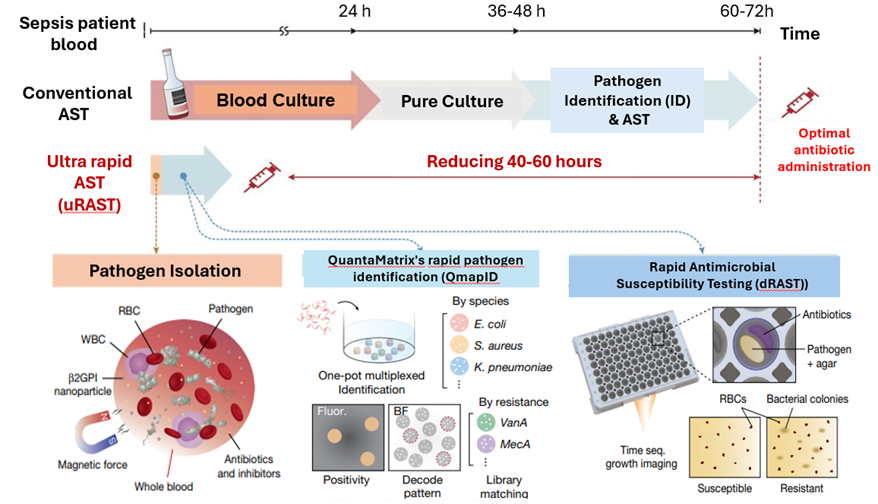
[Figure] Ultra-rapid antimicrobial susceptibility test (uRAST) process sequence
A new path has opened to dramatically improve the prognosis of sepsis, a fatal disease where every second of treatment counts. A joint research team from Seoul National University Hospital and Seoul National University recently introduced an antimicrobial susceptibility testing (AST) technology in the world's top academic journal 'Nature (IF;50.5)'. Using this technology, tests that would normally take 2-3 days can be completed in half a day, allowing for faster sepsis treatment.
Park Wan Beom (Infectious Diseases), Kim Taeksoo (Laboratory Medicine), and Kim Inho (Hematology and Oncology) of Seoul National University Hospital and Professor Kwon Sunghoon (Electrical Engineering) of Seoul National University announced on July 7th that the results of a clinical trial showing that the ‘ultra-rapid antimicrobial susceptibility test (uRAST)’ technology, developed in collaboration with QuantaMatrix Co., Ltd., shortens test times by an average of 48 hours compared to traditional methods.
Sepsis is a disease that causes inflammatory responses throughout the body due to pathogen infection, and the mortality rate rapidly increases by approximately 9% per hour, resulting in the deaths of 2 to 5 out of every 10 patients. For treatment, the optimal antibiotic must be prescribed quickly, but there was previously a problem that doing this required an extremely time-consuming antibiotic susceptibility test.
Traditionally, antimicrobial susceptibility tests require a pre-culture phase (blood culture + pure culture) of 36-48 hours to grow enough pathogens. Afterward, pathogen identification and susceptibility testing take another 24-36 hours to determine the effective antibiotic. In particular, ‘blood culture’, the initial stage of pre-culture, can take from 1 to 7 days depending on the growth rate of the pathogen, so shortening this stage was an important technical challenge for improving the prognosis of sepsis.
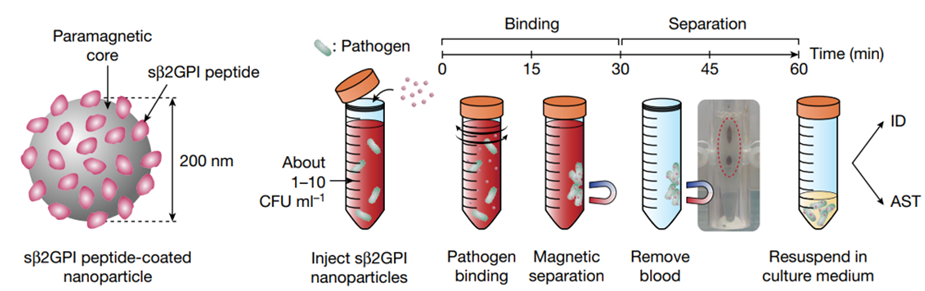
[Figure] Workflow of the pathogen isolation process using sβ2GPI nanoparticles
The uRAST developed by the research team is the world's first 'ultra-rapid antimicrobial susceptibility test' technology that skips the blood culture phase. Instead, It can directly separate pathogens from the blood by administering synthetic nanoparticles. These synthetic nanoparticles, sβ2GPI nanoparticles, are coated with innate immune substances, so they can recognize the common molecular structure of pathogens and attach to a wide range of pathogens. After that, if these nanoparticles are filtered out with a magnet, the majority of the pathogens in the blood can be extracted in 60 minutes.
Then, through a rapid 6-hour culture, a sufficient number of pathogens as required for the susceptibility test can be secured, reducing the pre-culture time that before took at least 36 hours and allowing rapid follow-up testing.
In addition, the research team introduced QuantaMatrix's rapid pathogen identification (QmapID) and rapid antibiotic susceptibility test (dRAST) into the pathogen identification and antimicrobial susceptibility testing process conducted after culture, reducing the existing time required from at least 24 hours to 6 hours.
As a result of conducting a clinical trial on 190 patients suspected of suffering sepsis infection, uRAST was shown to complete all tests ‘within 13 hours’ with only 10 ml of blood, significantly improving the testing time compared to existing equipment, reducing it by an average of about 48 hours. The research team emphasized that this is the fastest antimicrobial susceptibility testing technology demonstrated in the world to date.
Compared with the standard test method, uRAST was able to identify the bacteria with 100% accuracy at the pathogen identification stage, and the CA (Categorical Agreement) of the susceptibility test was 94.9%, which meets the FDA standard. This means that uRAST is not only rapid but also has a high level of accuracy similar to the standard method.
Professor Park Wan Beom (Infectious Diseases) emphasized, “Since the time required for antimicrobial susceptibility testing is long, unfortunately, many patients die because they are unable to receive the optimal antibiotics at the right time.” He continued, “uRAST, which enables ultra-fast antimicrobial susceptibility testing, will play an important role in increasing patient survival rates and further advancing sepsis treatment.”
Professor Kim Taeksoo (Laboratory Medicine) said, “uRAST technology, which integrates all necessary diagnostic testing processes within a short time after blood collection, is a groundbreaking advancement in the diagnosis of sepsis.” He continued, “We expect that uRAST will be utilized as a new medical technology that can quickly identify the type of pathogen and find effective antibiotics, thereby improving the prognosis of sepsis patients.”

[Pictures from left] Prof Park Wanbeom, Prof Kim Taeksoo, and Prof Kim Inho from SNUH and Prof Kwon Sunghoon from SNU