Development of a New Treatment for Refractory·Relapsed Diffuse Large B-Cell Lymphoma with Poor Prognosis
New Possibility for Treatment of Refractory·Relapsed Diffuse Large B-Cell Lymphoma
- Domestic research team announces phase 2 clinical trial results of combination therapy with BTK inhibitor, lenalidomide, and rituximab
- Objective Response Rate (ORR) 54.5%, Complete Response (CR) 31.8%, 1-year Progression-Free Survival (PFS) 33.1%
A new treatment for refractory/relapsed diffuse large B-cell lymphoma (DLBCL) with poor prognosis has been proposed. A domestic research team developed a new chemotherapy based on the targeted anticancer agent 'BTK inhibitor' used in the treatment of low-grade lymphoma.
This treatment showed a response in more than half of the patients, with complete tumor disappearance in 3 out of 10 patients. The research results were published in the international journal 'Nature Communications' (IF: 16.6).
Professors Koh Young-il and Park Chang-hee of the Department of Hemato Oncology at Seoul National University Hospital(SNUH), along with the Korean Lymphoma Clinical Study Consortium (CISL) joint research team, designed the combination therapy with BTK inhibitor, lenalidomide, and rituximab for the treatment of refractory/relapsed DLBCL, and confirmed its efficacy and safety through a single-arm phase 2 clinical trial, announced on the 23rd.
'Diffuse Large B-Cell Lymphoma (DLBCL)' is a type of aggressive lymphoma that progresses rapidly, and more than half of malignant lymphomas are of this type. It is treated with chemotherapy combining anticancer drugs like rituximab, but 4 out of 10 patients do not respond to the initial treatment or experience relapse.
Although the introduction of CAR-T therapy has improved the prognosis for these patients, about half of the refractory/relapsed patients still lack an established standard treatment, and their expected survival is only 6 months, indicating a poor prognosis. Thus, a new treatment for these patients was needed.
The research team developed the R2A regimen, combining the targeted anticancer agent 'BTK inhibitor (acalabrutinib)', the immunomodulatory anticancer agent 'lenalidomide' used in the treatment of multiple myeloma, and the C20-targeted anticancer agent 'rituximab'. They then conducted a single-arm phase 2 clinical trial, administering this regimen to 66 patients and monitoring the treatment response.
After an average follow-up of about 9 months, the objective response rate (ORR) was 54.5%, with more than half of the patients showing a reduction in tumor size or complete disappearance of the tumor. Particularly, the complete response (CR) rate was 31.8%, with 3 out of 10 patients achieving complete tumor disappearance.
Additionally, the 1-year progression-free survival (PFS) rate was 33.1%, with 1 out of 3 patients showing no tumor progression for a year.
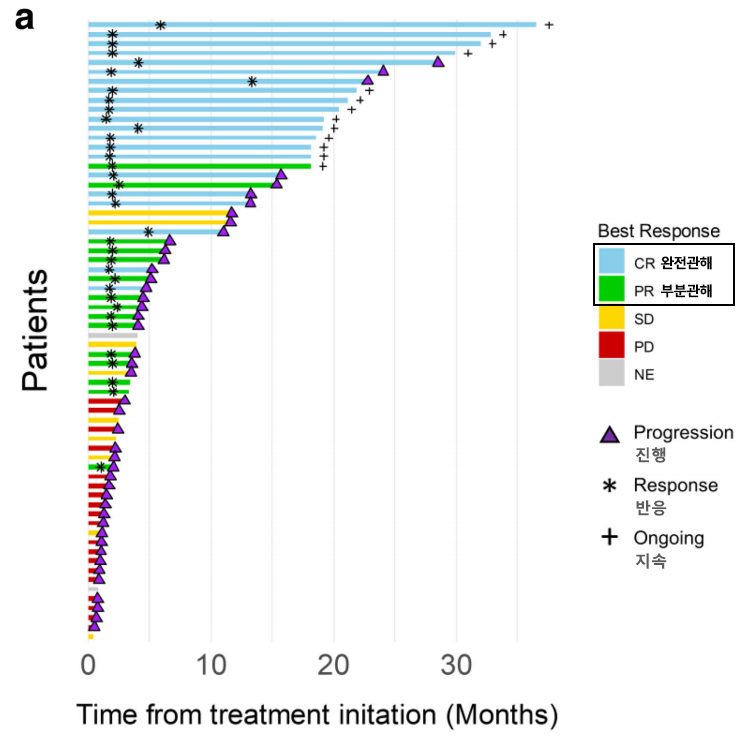
[Graph] The objective response rate (ORR) of the R2A regimen is 54.5%, with 36 out of 66 patients showing a treatment response. Notably, 21 patients (31.8%) achieved complete response (CR), with many maintaining remission without tumor progression.
Based on these results, the research team emphasized that the BTK inhibitor is effective not only in low-risk lymphomas but also in the treatment of aggressive lymphomas, and that combination therapy based on this anticancer agent could be a new approach to curing refractory/relapsed DLBCL.
Furthermore, additional biomarker analyses based on DNA, RNA, and protein were conducted to identify the patient group that responds best to the R2A regimen. It was found that patients with MYD88 mutations or activated NF-κB protein activity showed significant treatment responses.
Professor Koh Young-il stated, "BTK inhibitor-based anticancer therapy will play an important role in the treatment of refractory/relapsed DLBCL patients who have failed CAR-T therapy," adding that "combining the R2A regimen, verified in this study, with recently developed bispecific antibody therapy and CAR-T therapy could develop another treatment to improve survival rates."

[Photo from left] Professor Koh Young-il, Park Chang-hee of the Department of Hemato Oncology at SNUH