SHUN, the first to identify the mechanism by which a high-calorie diet causes diabetes
- Professor Kim Hyo-Soo's team: a high-calorie diet → ↑the amount of Resistin → mitochondrial division ↑ → ATP production↓→ Mitochondrial function↓ → Insulin resistance↑ → Investigation of the development of diabetes
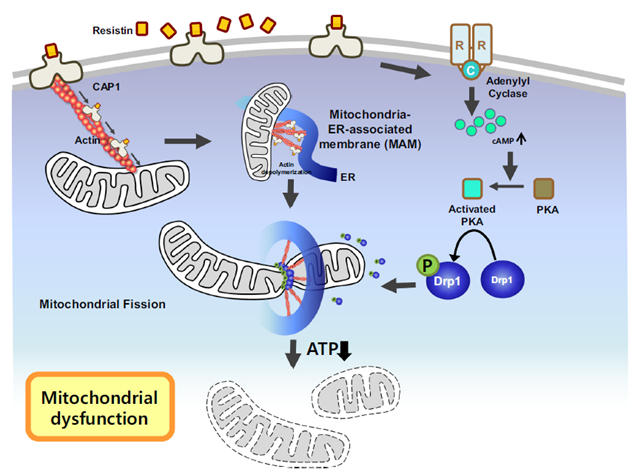
[Figure] Schematic figure of our proposed mechanism for dysregulation of mitochondria dynamics by resistin treatment
From the knowledge that a high-calorie diet can lead to diabetes, researchers at Seoul National University Hospital have identified, for the first time in the world, that Resistin causes diabetes by decreasing mitochondrial function.
In other words, a new mechanism suggested that Resistin increased due to structural transformations induced by high-calorie meals and functional degradation of mitochondria through interaction with its receptor, CAP1, thereby reducing the production of ATP, an energy source, and causing diabetes.
A research team led by Professor Kim Hyo-Soo (Professor Yang Han-Mo, Research Professor Kim Joonoh) of the Department of Cardiovascular Medicine at SNUH announced that they had confirmed this fact by investigating whether Resistin, an adipokine related to insulin resistance, damages mitochondrial homeostasis and causes metabolic disorders.
Mitochondria are important organelles within cells that generate energy for cell activity. It is known that abnormalities in this function cause diabetes, metabolic syndromes, and degenerative brain diseases. It is also known that obesity, which is a root cause of adult diseases, causes mitochondrial dysfunction.
The research team paid attention to ‘Resistin’, a hormone secreted from fat cells in mice that induces insulin resistance, but in humans, a cytokine secreted from white blood cells known to cause chronic inflammation. So far, the causal relationship between Resistin and diabetes in humans has not been known.
The research team divided their investigation into two groups: ▲humanized resistant mice (genetically engineered mice that hyper-secrete human Resistin) and ▲control group (Resistin-knockout mice), then fed them a high-calorie diet for 3 months and studied the structure of mitochondria in muscles using an electron microscope.
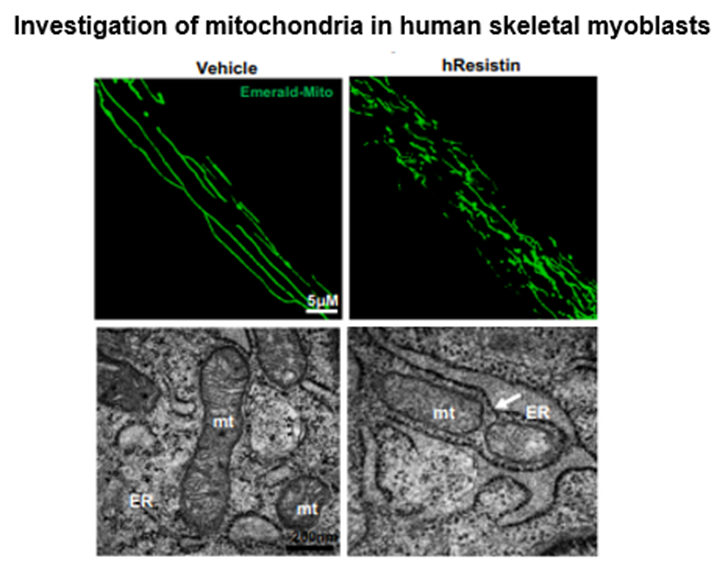
[Figure] Resistin-treated human myoblasts show me an enhanced mitochondrial dysfunction by fission. The white arrow indicates the site of mitochondrial fission.
Under a high-calorie diet, mitochondria in Resistin-knockout mice maintained a normal state, whereas, in humanized Resistin mice, mitochondrial fragmentation was abnormal. In other words, the research team discovered that Resistin causes mitochondria dysfunction, and went on to study this mechanism using human skeletal muscle cells.
Resultantly, they found that Resistin binds to the CAP1 receptor on the surface of skeletal muscle cells, moves to the mitochondria in the cell, forms MAM (the membrane connecting mitochondria and the endoplasmic reticulum), constricts mitochondria, and activates the PKA signaling pathway. It was demonstrated that phosphorylation and activation of the Drp1 protein, which is important for mitochondrial division, ultimately destroys the structure of mitochondria.
Moreover, the reduction in the production of ATP, an energy source, was demonstrated by measuring the oxygen consumption of muscle cells in real-time. In other words, it was proved that human Resistin induces mitochondrial division and destroys its structure, thereby reducing function and inhibiting ATP generation, and as a result, glucose use in muscle cells decreases, leading to diabetes. It was proved that mitochondrial dysfunction in muscle causes insulin resistance in mice and leads to the aggravation of diabetes.
Next, the research team analyzed genetically modified mice lacking the CAP1 gene, a receptor for Resistin, to verify whether the harmful event could be prevented by blocking Resistin. It was also found that in these mice, because Resistin could not bind to the receptor, the adverse effects of Resistin were blocked, preventing mitochondrial dysfunction even under a high-calorie diet. At the same time, the effect of preventing diabetes was also confirmed.
The research team developed a peptide that inhibits the binding of Resistin and CAP1 to explore the possibility of treatment and proved that this peptide treatment prevents mitochondrial dysfunction and prevents diabetes even when combined with a high-calorie diet.
Professor Kim Hyo-Soo said, “Through this study we observed that when you have a high-calorie meal, the amount of Resistin increases, and as a result, Resistin binds to the CAP1 receptor on the cell surface to destroy mitochondria, thereby inhibiting the function of muscle cells. By doing so, it was identified that it causes diabetes.”
“Conclusively, in conjunction with previously published results, it suggests that the Resistin-CAP1 conjugate can be a promising target for the development of therapeutic agents for metabolic diseases such as diabetes and fatty liver,” he added.
“Currently, we have developed an antibody that alleviates inflammation by inhibiting the interaction between Resistin and its receptor, CAP1 protein,” he continued. “We are developing this as a new treatment for metabolic diseases and inflammatory bowel disease.”
This study was carried out with the support of the research-oriented hospital project funded by the Ministry of Health and Welfare, and the results of the study were published in the online edition of Metabolism (METABOLISM: Clinical and Experimental, IF: 13.9), a world-renowned academic journal in the field of metabolic disease.
[Pictures from the left] Department of Cardiology Prof Kim Hyo-Soo, Prof Yang Han-Mo and Dr Kim Joonoh