Professor at Seoul National University Hospital succeeds in developing a new standard treatment for advanced biliary tract cancer
- Department of Hemato Oncology, Prof Oh Do-Youn, found success as the chief researcher of global phase 3 clinical research
- The risk of dying from advanced biliary tract cancer can be lowered by 20% with the current combination of standard chemotherapy and immune chemotherapy.
The standard treatment for inoperable, advanced biliary tract cancer is expected to change for the first time in 10 years. The results of the first global Phase 3 clinical study that suggested a new standard of treatment using a combination of standard anticancer drugs and immunotherapy were recently published. Compared to standard chemotherapy, the new treatment reduced the risk of death in patients with advanced biliary tract cancer by 20% and increased their long-term survival rate.
The results of this study were presented at the American Society of Clinical Oncology Symposium (ASCO GI 2022) held on the 20th and 22nd. These results came from AstraZeneca's global phase 3 clinical study (TOPAZ-1), which started and expanded from a phase 2 clinical study led by a domestic researcher. The standard treatment paradigm for advanced biliary tract cancer around the world has been changed by a Korean research team.
The overall lead researcher for this global phase 3 clinical study is Prof Oh Do-Youn from the Hemato Oncology department, Seoul National University Hospital. In a situation in which the development of a new treatment is desperately needed, this achievement reached through the hard work and dedication of Professor Oh has finally brought new hope to patients with biliary tract cancer.
Biliary tract cancer is a cancer that ranks 9th to 10th in prevalence in Korea. It is cancer with a higher prominence in Korea than in the West, and its incidence is increasing worldwide. Many patients with biliary tract cancer are diagnosed at an advanced stage where surgery is difficult, and even after surgery, many recurrences occur. In this case, a cure is impossible, so chemotherapy is required to prolong their survival period.
However, truly effective therapeutics worldwide have been limited. Since 2010, the first-line treatment for advanced biliary tract cancer has been cytotoxic chemotherapy (Gemcitabine + Cisplatin). Despite the median survival period of less than one year for this treatment, this cancer treatment had to be continued as the standard treatment around the world because no better alternative has been developed over the past decade.
In order to improve the therapeutic effect of biliary tract cancer, Professor Oh Do-Youn conducted a researcher-led phase 2 clinical study using combinational therapy consisting of current standard anticancer drugs and immunotherapy (Durvalumab, Tremelimumab). As a result, she confirmed that it had an encouraging anti-tumor effect and had no cause for concern in terms of side effects.
Based on these positive research results, Professor Oh started a global phase 3 clinical study with AstraZeneca to compare the effects of the current standard chemotherapy and chemotherapy + Durvalumab combination therapy. This study was performed as a randomized, double-blind, placebo-controlled, multicenter, multinational trial to evaluate safety and efficacy.
A total of 685 patients with advanced and recurrent biliary tract cancer were enrolled in 17 countries, including Asia, the United States, Europe, and South America, and more than half (about 54%) of the patients were from Asian countries.
685 patients were randomized at a 1:1 ratio into a ▲ Chemotherapy + Durvalumab combination group (341 patients) and ▲ Chemotherapy + Placebo combination group (344 patients) and accordingly received treatment.
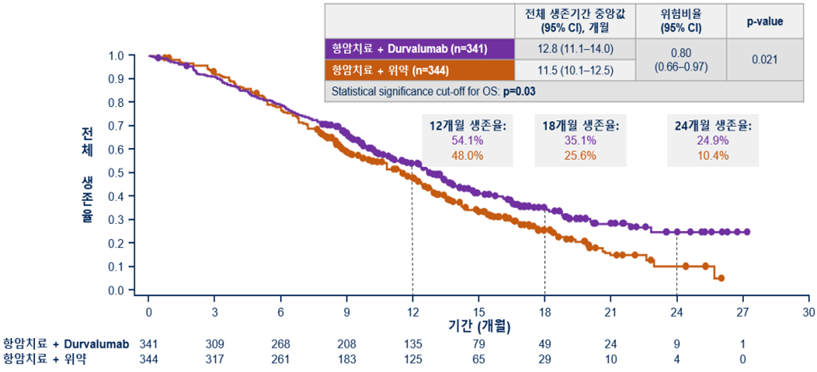
[Figure] Overall survival period (20% lower risk of death in Durvalumab combination group)
As a result of interim analysis at pre-planned time points, it was confirmed that the duration of overall survival (the period from study enrollment to death) was significantly prolonged in the Durvalumab combination group compared to the Placebo combination group. The risk of death was 20% lower in the Durvalumab combination group.
The median overall survival time was 12.8 months in the Durvalumab group compared to 11.5 months in the Placebo combination group.
In particular, the effect of the Durvalumab combination on the improvement of survival increased over time, and the 2-year survival rate was 24.9% and 10.4% in the Durvalumab combination group and the Placebo combination group, respectively, showing an absolute survival rate improvement of about 15%.
Median progression-free survival (period from enrollment to cancer progression) was also improved to 7.2 months in the Durvalumab combination group compared to 5.7 months in the Placebo combination group, reducing the risk of cancer progression by 25%.
The objective response rate (proportion of patients with a 30% or greater reduction in cancer size) improved to 26.7% in the Durvalumab combination group compared to 18.7% in the Placebo group.
In addition to these improved effects, there was little difference in the incidence of side effects between the two groups, and no new serious side effects were observed.
Professor Oh highlighted the significant achievement of this research as the following: it can deliver hope to patients with biliary tract cancer being the first global Phase 3 clinical study that has demonstrated an improvement in survival compared to standard chemotherapy, which has been established as the primary treatment for advanced biliary tract cancer for the past 10 years.
In addition, Professor Oh emphasized that it is the first phase 3 clinical study to prove the success of immune-cancer-therapy in biliary tract cancer, where before there was relatively little opportunity for new drug development. Henceforth, she expects more active research progress on the development of new therapeutics on biliary duct cancer based on her research.
Professor Oh Do-Youn said, “After confirming the possibility of developing a new treatment through the researcher-led phase 2 clinical study, we led the global phase 3 clinical study based upon it, led by pharmaceutical companies. Speaking as a Korean researcher myself, the fact that the development of the new therapeutic was successful is very meaningful to me, especially having played a leading role in the project, as well as to all Korean researchers.”
She also added, “We expect that the treatment proven through this study will establish a new standard treatment for patients with advanced biliary tract cancer worldwide.”

[Picture] Department of Hemato Oncology, Professor Oh Do-Youn