Pediatric leukaemia CAR-T medicinal treatment to be produced in hospital for the first time in Korea
- Approved Ministry of Health and Welfare and Ministry of Food and Drug Safety, with clinical research now scheduled to start
- Better access to patients and shorter turnaround times
On the 8th of December 2021, Seoul National University Hospital received approval for the ‘CAR-T treatment clinical study for pediatric acute lymphoblastic leukaemia’ to be carried out.
CAR-T therapy is a treatment in which immune cells (T cells) from a patient's blood undergo genetic manipulation so that they can better recognize cancer, then are cultured before being put back into the patient's body. These immune cells precisely target cancer cells while minimizing damage to normal cells in the body. So CAR-T therapy have been attracting attention as an innovative and cutting-edge treatment.
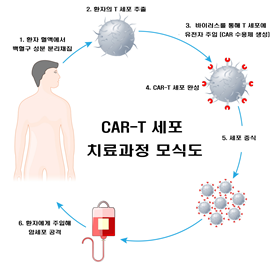
1. Separation of leukocyte components from patient blood
2. T-cell extraction from patients
3. Gene injection into T-cell via virus (CAR-Receptor formation)
4. CAR-T cell production
5. Cell proliferation
6. Cancer cells attack cancerous cells after injection into the patient
[Figure 1] Schematic diagram of CAR-T Cell treatment process
This approval was achieved only about eight months after the submission of the clinical research plan. With the implementation of the Advanced Regenerative Bio Act, Seoul National University Hospital submitted a clinical research plan for ‘Hospital-produced CAR-T treatment’ for children with leukaemia in April 2021. After deliberation by the Ministry of Health and Welfare and the Ministry of Food and Drug Safety, this plan was approved on December 8 as the first high-risk, advanced regenerative medicine clinical study in Korea.
It is expected to be a hope for patients, with full-scale clinical research targeting child/adolescent patients with relapsed/refractory acute lymphoblastic leukaemia. Existing overseas CAR-T treatments can cost 500 million won per treatment, making it difficult for patients to access them. Now the clinical study at Seoul National University Hospital has been officially approved, patients are able to receive free CAR-T treatment products provided by the hospital.
This treatment is excellent not only in cost but also in speed. Existing CAR-T treatment takes an average of three weeks as the patient's T cells need to be sent overseas, be left to proliferate and then be re-injected. Since this CAR-T is being produced in-hospital, it has dramatically reduced times to an average of 12 days. It is expected that children with acute lymphoblastic leukaemia, who can struggle with long waiting periods, will be able to receive prompt treatment.
This is the first time for a domestic hospital to produce and conduct clinical research on its CAR-T treatment. As a first-time study that had never been done before, it went through rigorous evaluation by the Ministry of Health and Welfare and the Ministry of Food and Drug Safety, both of which recognized the research capacity, safety, and efficacy of the study.
Professor Kang Hyung-Jin of the Department of Pediatrics at Seoul National University Hospital, who is in charge of the study, said, "I am glad that the research plan was approved after careful deliberation." However, he also stated, “As this is the first study of its subject in Korea, it has undergone a long period of scrutiny, and I feel sorry for the children who could not receive treatment over this time. We hope that this study will provide hope to children and adolesents with leukaemia in Korea who have not before been able to receive CAR-T treatment due to cost burden.”
Additionally, this study was prepared over the past 4 years by Professor Kang Hyung-Jin in the 'biotherapy' fostering unit of the Seoul National University Hospital Research Center Program, for which Professor Hyo-Soo Kim of Seoul National University Hospital is responsible.
SNUH is preparing a 'CAR-T development one-stop system' that can perform ‘GMP production- preclinical trials-clinical trials’ all at once by utilizing hospital assets. Therefore, CAR-T therapeutics developed by domestic researchers can be easily applied to patients. Beyond the CAR-T treatment for pediatric leukaemia, SNUH also plans to make efforts to develop CAR-T treatment for other various diseases.
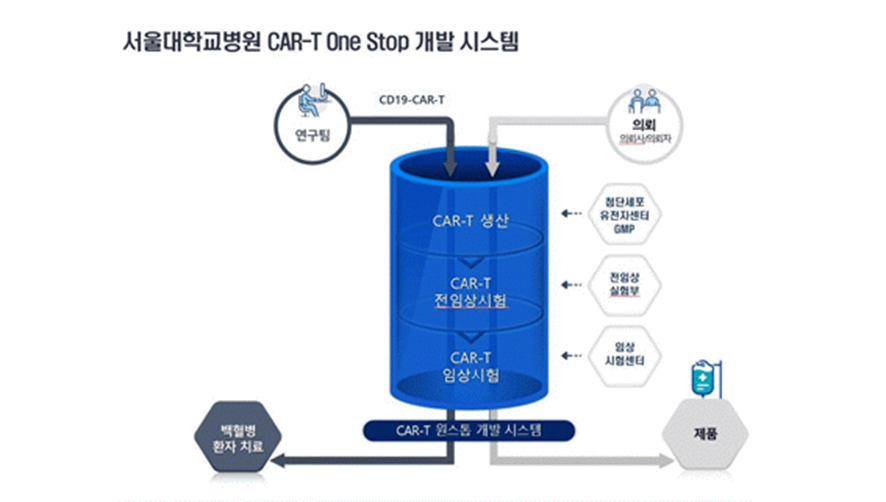
Research team (CD19-CAR-T) Request (client)
CAR-T Production <-- Advanced Cell Gene Center GMP
CAR-T Preclinical Trial <-- Preclinical Lab
CAR-T Clinical Trial <-- Clinical Trial Center
CAR-T One-Stop developmental system
Leukaemia patient treatment Products (Therapeutics)
[Figure 2] System of SNUH CAR-T One-Stop development